Abstract
BACKGROUND
Severe cytopenias are common complications after CAR T-cell therapy. Up to 25% of these patients may have prolonged cytopenias for more than three months, under mechanisms not well characterized and with scarce therapeutic options. We aim to describe these cytopenias during the first year after CAR T-cell in patients with large B-cell lymphoma and identify risk factors for their development.
MATERIAL AND METHODS
We included adult recipients of CD-19 CAR T-cell for large B-cell lymphoma. Using CTCAE version 5, grade 4 severe cytopenias were defined as: hemoglobin < 6.5 g/dL, platelets < 25.000/mm3 and neutrophils < 500/mm3. Cytopenias were studied by cumulative incidence, with relapse/progression, death, and the beginning of new chemotherapy used as competing events.
RESULTS
We included 199 patients treated with CD-19 CAR-T (axicabtagene-ciloleucel [n=100, 50%], tisagenlecleucel [63, 32%] and lisocabtagene-maraleucel [36, 18%]). Most patients were heavily pre-treated (> 3 treatment lines [82, 41%]) including 4.5% (n=9) and 22% (44) patients who had received previous allogeneic and autologous stem cell transplantation, respectively. Systemic bridging therapy was used in 59% of patients and 26% had bone marrow involvement of their lymphoma before lymphodepletion.
Post-CAR-T CRS was observed in 74% (148) of the patients with grade >3 in 6.5% (13). Overall incidence of ICANS was 30.5% (61) and grade >3 12.5% (25). With a median follow-up of 27.6 months, the median overall survival was 21 months (95% IC: 14, not reached) and the median progression-free survival was 6 months (95% IC: 3.4-9.3). Best response at day 100 was 56% complete response, 21% partial response and 24% stable/progressive disease.
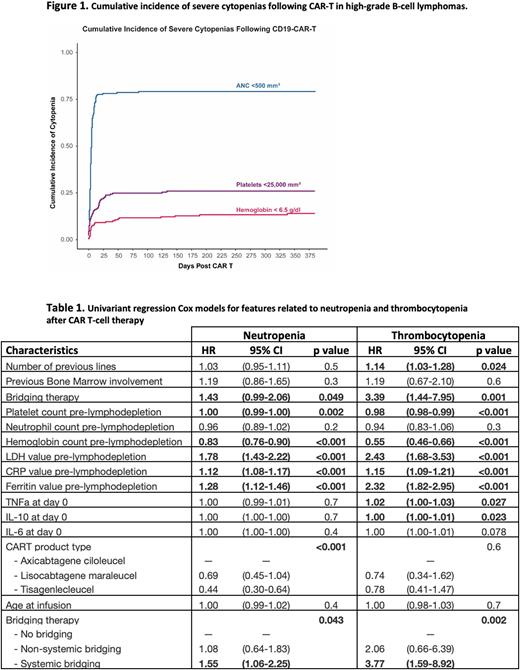
The cumulative incidence (CI) of neutropenia was 78% within the first 30 days after CAR-T. To estimate late neutropenia, we performed a landmark analysis at multiple timepoints and found a cumulative neutropenia incidence of 8.1% between 30 and 90 days and 5.3% between 90 and 365 days after CAR-T. The CI of thrombocytopenia was 24%, 25% and 25% for the first 30 days, 30-90 days, and 90-365 days respectively. The CI of anemia was 9.6% during the first month (Figure 1).
We sought to identify baseline features associated with the development of severe cytopenias. Pre-lymphodepletion markers, including baseline values of hemoglobin, platelet, LDH, markers of inflammation (CRP, ferritin), and the requirement for systemic bridging therapy were significantly associated (p< 0.05) with both neutropenia and thrombocytopenia in univariable Cox regression models (Table 1). In addition, the type of CART product was also related to neutropenia; patients treated with axicabtagene ciloleucel were more likely to experience neutropenia (Tisagenlecleucel vs. Axi-cel, HR 0.44 [95% CI: 0.30-0.64]). Interestingly, elevated inflammatory cytokines at day 0 (measured before infusion), such as IL10 and TNFα were associated with the development of thrombocytopenia (p = 0.027 and p = 0.023, respectively).
CONCLUSION
Severe cytopenias following CD19-CAR-T are common in the real-world setting, with an estimated incidence of 25% and 80% incidence of profound thrombocytopenia and neutropenia, respectively. Our data suggest that clinical features, baseline counts, and the inflammatory profile before infusion are important determinants of cytopenia risk in CAR-T recipients.
Disclosures
Shouval:MyBiotics: Consultancy; Medexus: Consultancy, Ended employment in the past 24 months. Batlevi:Bayer: Research Funding; ADC Therapeutics: Other: Provision of Services; Dava Oncology: Other: Provision of Services; Roche/Genentech: Research Funding; Novartis: Research Funding; Janssen: Research Funding; Epizyme: Research Funding; Bristol-Myers Squibb: Other: Ownership / Equity Interests; Provision of Services; Autolus: Research Funding; Xynomic: Research Funding; GLG Pharma: Consultancy; Juno/Celgene: Consultancy; Kite Pharma: Consultancy; Life Sciences: Consultancy; Seattle Genetics: Consultancy. Palomba:Da Volterra: Honoraria; Garuda: Honoraria; GSK: Honoraria; Lygenesis: Honoraria; Ceramedix: Honoraria; Thymofox: Honoraria; Pluto Therapeutics: Current holder of stock options in a privately-held company, Honoraria; Notch Therapeutics: Current holder of stock options in a privately-held company, Honoraria; Nektar Therapeutics: Honoraria; Frazier Healthcare Partners: Honoraria; Rheos: Honoraria; Vor Biopharma: Honoraria; Seres: Current holder of stock options in a privately-held company, Honoraria, Research Funding; MustangBio: Honoraria; Kite: Honoraria; BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Synthekine: Honoraria; Novartis: Honoraria; BMS: Consultancy. Shah:Amgen: Research Funding; Beyond Spring: Research Funding; Janssen: Research Funding. Salles:AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, a Gilead Company, Miltenyi, MorphoSys, Takeda, and VelosBio: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys AG, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol-Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, Kite, a Gilead Company, Loxo, Rapt: Consultancy; Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys AG, Amgen, Bayer, Epizyme, Regeneron, Kite, a Gilead Company: Honoraria. Scordo:i3Health (CME): Honoraria; Amgen, Inc.: Research Funding; Kite - A Gilead Company: Other: Ad-hoc advisory board (past); McKinsey & Company: Consultancy; Angiocrine Bioscience, Inc.: Consultancy, Research Funding; Medscape, LCC (CME): Honoraria; Omeros Corporation: Consultancy, Research Funding. Perales:VectivBio AG: Honoraria; DSMB: Other; Orca Bio: Consultancy; Omeros: Consultancy; Vor Biopharma: Honoraria; Cidara Therapeutics: Consultancy; Sellas Life Sciences: Consultancy; Abbvie: Honoraria; Astellas: Honoraria; Celgene: Honoraria; Karyopharm: Honoraria; MorphoSys: Consultancy, Honoraria; Takeda: Honoraria; Nektar Therapeutics: Consultancy, Honoraria; Medigene: Consultancy; Servier: Consultancy; Bellicum: Honoraria; Novartis: Honoraria; Miltenyi Biotec: Consultancy, Honoraria; Merck: Consultancy; Kite, a Gilead Company: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Bristol-Mysers Squibb: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal